CUDA Spotlight: Mark BatheBy Calisa Cole, posted June 12, 2014  GPU-Accelerated Structural Nucleic Acid NanotechnologyProf. Mark Bathe is an Associate Professor of Biological Engineering at the Massachusetts Institute of Technology in Cambridge, Massachusetts. His lab focuses on in silico design and programming of synthetic nucleic acid scaffolds for engineering light-harvesting antennas, multi-enzyme cascades, cellular delivery vehicles, and fluorescent biomolecular probes, which he assays using innovative quantitative imaging techniques. Q & A with Dr. Mark BatheNVIDIA: Mark, tell us about your work with structural nucleic acids and DNA nanotechnology. A lesser known, powerful alternative use for DNA is that of a programmable structural element for engineering molecular scaffolds of precise shape and size at the nanometer-scale. 
This molecular engineering paradigm dates back to early work by Nadrian Seeman in the 1980s, when he demonstrated theoretically that DNA could be programmed to form large-scale synthetic assemblies due to its unique and highly specific basepairing properties. Since that landmark work, the field of molecular engineering using nucleic acids has witnessed explosive growth. Unlike proteins, DNA is highly programmable structurally because it can be designed to robustly self-assemble into large-scale molecular architectures of precise nanometer-scale structural features, dimensions, and mechanical properties. These assemblies can subsequently be functionalized chemically using lipids, dyes, and proteins for diverse applications in biomolecular science and technology. The rapidly decreasing cost of synthetic DNA, together with rational computational design rules, now enable a plethora of structured nanoscale materials to be designed, with the ultimate aim of replicating the function of biological protein assemblies that have evolved over billions of years. NVIDIA: What are some of the potential applications?
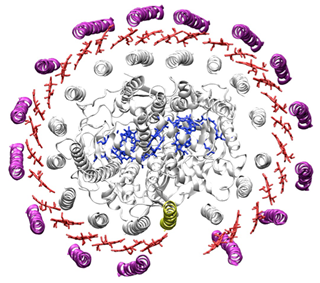
Potential applications are truly countless, limited only by our imagination as biomolecular engineers, as well as the reality of the fact that this field is still in its relative infancy given that we're still in early stages of understanding how to endow these assemblies with important chemical, biological, photonic, and excitonic properties. Nevertheless, there is so much energy and effort focused in this area across diverse research groups worldwide that it is very likely that this innovative approach to nanobiotechnology will succeed to make major breakthroughs in the coming decade. NVIDIA: Why is GPU computing important to your work? Second, we utilize GPU computing for the calculation of excitonic properties of DNA-scaffolded chromophore assemblies, in collaboration with Alan Aspuru-Guzik at Harvard whose expertise is in the calculation of excitonic properties of bacterial photosynthetic complexes. Alan's group has simulated excitonic properties of the light-harvesting complex from green sulfur bacteria, and we're also very interested in the light-harvesting properties of the antenna complex from purple bacterium, extensively analyzed by the Schulten group at UIUC (learn more here (pdf) and here (pdf)).
Bacterial light-harvesting complex (Richter et al PNAS 2007) Finally, as our molecular modeling and structure-prediction frameworks mature, we envision an increasing role of GPUs in crowd-sourcing both exploration of novel functional nanoscale materials, as well as in optimizing existing ones. For example, crowd-sourcing innovation in molecular design has been very successfully implemented by David Baker's lab for proteins in FoldIt and by Rhiju Das' lab for RNAs using EteRNA, and we envision a similar implementation for synthetic DNA assemblies being of major use to drive innovation and deeper understanding of successful design principles in our field. NVIDIA: What are some of the biggest challenges you are tackling? In addition, we seek to encode the rules of 3D DNA structure prediction from sequence in our computational modeling framework CanDo to turn this molecular design paradigm into a robust molecular engineering framework. Enabling general 3D structure prediction from DNA sequence will enable researchers worldwide to leverage this unique bio-nanotechnology platform for their own research and application purposes, which should dramatically accelerate advances in this area. The uniqueness of our computational approach is that we do not simulate the actual self-assembly or folding process of DNA, but rather directly solve for the ground-state structure based on internal topological constraints that are imposed by programmed complementary DNA sequences. CanDo currently operates principally on finite element software, which is also increasingly being driven by GPU computing. Parameterization of our model and structure-prediction procedure, currently implemented in the commercial program ADINA, is performed using a combination of experiment and large-scale molecular dynamics simulations driven by NAMD, which is greatly enabled by GPU-accelerated computing for long time-scale all-atom simulations of DNA structure and dynamics.
Our mission is to transform structural DNA programming from the design of detailed sequence-level base-pairing, which is a bit like assembly language used in computers in the 60s and 70s, to a higher-level C++, Java, or most recently iPhone app-like language so that anyone can write their own functions to program complex DNA nanostructures, greatly broadening participation in this technology. Enabling programmed bacterial production of DNA as raw material for structured nucleic acid assemblies is another major challenge that some of our colleagues in cell-based synthetic biology are tackling and best suited to advance, which would be a major boon for the field because we could finally envision large-scale raw DNA material production for diverse nanoscale biomaterials applications. Again, integrating proteins that harbor light-harvesting, enzymatic, and other chemical functionality to co-assemble with DNA structures inside and outside of cells has the capacity to eventually translate this rational bottom-up materials design approach to commercial applications. And while DNA is a super attractive raw material itself, we can easily envision other, nucleotide analogues eventually being used instead. DNA may eventually only end up being the inspiration for what will be a highly versatile biologically-based polymer for programming nanostructured materials of diverse functionalities. NVIDIA: You have a very inter-disciplinary background. How does this benefit your research? The Department of Biological Engineering at MIT is a wonderful example of this, where faculty and students come from all backgrounds to work together on fundamental problems in biologically-based and –inspired science and technology, using diverse tools and approaches that suit their needs from a combination of biology, chemistry, physics, math, computation, and engineering to tackle important, major long-term challenges in energy, medicine, and biomaterials, amongst other important areas. This environment has been the perfect venue for us to leverage diverse approaches and backgrounds to impact the areas we're pursuing, though we're only at the very beginning of our journey. It's quite fascinating how rapidly traditional boundaries between classical disciplines and majors are disintegrating, largely due to major technological advances across computation, materials, biotechnology, and synthesis, which the internet is also compounding in an excellent way by enabling both long-distance collaboration between groups with disparate backgrounds/expertise, and also making information readily available online. While this transformation presents tremendous opportunity for academic researchers, we also face a very important educational challenge in the coming decade in how to best train our undergraduate and graduate students to embrace and leverage this exciting transformation in technology and communication that we're witnessing! NVIDIA: What are you looking forward to in the next five years, in terms of technology advances? More recently, however, our ability to write or synthesize DNA is also increasing at a dramatic rate. In the next five years we hope that we can translate this ability to synthesize DNA at an ever-decreasing cost to enable researchers from many disciplines to directly write structured nucleic acids for diverse applications in nanoscale materials engineering. Nature has evolved a fascinating, sophisticated ability to assemble complex functional biomolecular assemblies using proteins, which we are optimistic that we will learn to mimic by functionalizing addressable DNA scaffolds of precise 3D nanometer-scale structure. While ambitious in a five-year time-frame, this ability would undoubtedly be transformative, endowing us with the nanometer-scale biomaterial equivalent of macroscopic engineering that is achieved using conventional materials such as metals, polymers, wood, etc. to fabricate macroscopic structural assemblies including cars, planes, homes, and buildings. Integrating smart sensing and response capabilities into biologically-based materials synthesized from DNA is an additional, longer-term aim that is also very exciting to think about. For now we're focused on using in silico modeling and analysis tools together with experimental characterization of DNA-scaffolded multi-chromophore and multi-enzyme assemblies to guide the design of functional nanoscale materials with novel excitonic, photonic, and chemical properties that cannot be replicated by any other molecular synthesis technique. If we're successful in implementing predictive models of DNA scaffold structure and function in silico, this will help bypass time consuming and costly experimental synthesis and characterization that is currently a bottleneck for advancing the discovery of next-generation functional nanoscale materials. A parallel effort is ongoing in the pharmaceutical industry, where companies seek to move medicinal chemistry in silico to screen computationally for functional therapeutic molecules that are otherwise extremely expensive and time-consuming to synthesize and test experimentally. 
While these efforts to translate materials discovery and design in silico require significant upfront investment, their pay-off is potentially enormous if we're successful, due to the major limitations associated with experimental synthesis and characterization. This is in line with the vision of the Materials Genome Initiative, though time will tell how successful we will be in the realm of DNA-based functional materials. NVIDIA: Who are some of your key collaborators? We've additionally been very fortunate to receive core ONR funding for our program both for personnel and a major high performance computing cluster through the ONR DURIP program, as well as NSF funding more recently through the Designing Materials to Revolutionize and Engineer our Future program, which funds our basic research into the in silico design and assembly of structured DNA assemblies, in collaboration with Peng Yin at Harvard Medical School and Hao Yan at ASU. We're also very excited to have recently been awarded a prestigious Human Frontiers Science Program grant to design DNA-scaffolded protein assemblies to enable cellular delivery of molecular payloads, with important long-term implications for targeted delivery of therapeutics to cancer cells in a collaborative project with Yamuna Krishnan at the University of Chicago, Ludger Johannes at the Curie Institute, and John Ipsen at the University of Southern Denmark. Finally, we receive generous support from NSF's Physics of Living Systems program that funds important cell biological work in our group directed towards using high-resolution fluorescence imaging to extract quantitative molecular properties from living systems, which we interrogate using innovative DNA-based molecular probes. We're currently combining these DNA-based molecular probes together with advanced fluorescence imaging and analysis techniques to probe neuronal synapse structure and dynamics involved in learning and memory, which is of major importance to understanding the development of neurological disorders such as Autism. From an outside perspective these research areas and applications appear quite diverse, but internally there's tremendous and growing synergy in our ability to program and probe biomolecular systems using a combination of innovative DNA-based devices and quantitative fluorescence imaging and spectroscopy. Bio for Mark BatheProf. Bathe obtained his Bachelor's, Master's and Doctoral Degrees at MIT working in the Departments of Mechanical Engineering, Chemical Engineering, Chemistry, and Biological Engineering before moving to Munich to carry out his postdoctoral research in Biological Physics. He returned to MIT in 2009 to join the faculty in the Department of Biological Engineering, where he runs an interdisciplinary research group consisting of Biologists, Chemists, Physicists, and Engineers focused on fundamental applied problems in computational biology and biophysics. Relevant Links Contact Info # # # |